Abstract
Background Anti-CD20 mAbs are an important element in the therapeutic armamentarium for B-cell malignancies such as chronic lymphocytic leukemia (CLL). Although new targeted drugs (Ibrutinib & Idelalisib) and recently developed anti-CD20 mAbs (obinutuzumab & ofatumumab) have significant activity, the clinical management is limited by toxicity or low sensitivity to anti-CD20 mAbs. Herein we propose Drug-Free Macromolecular Therapeutics (DFMT) as a novel class of therapeutics that amplifies CD20 crosslinking and triggers apoptosis. The unique features of DFMT include augmentation of multivalent CD20 crosslinking, immune effector independence and no cytotoxic agents. Its effectiveness has been demonstrated in vitro and in preclinical Non-Hodgkin Lymphoma (NHL) models including a CD20-deficient rituximab (RTX)-resistant model, in which DFMT combined with a long-circulating polymer-gemcitabine conjugate (2P-GEM), induced prolonged survival and tumor clearance from bone marrows.[Zhang R, et al. PNAS 2014; Li L, et al. ACS Nano 2018] Therefore, we conducted assessment of DFMT on patient samples.
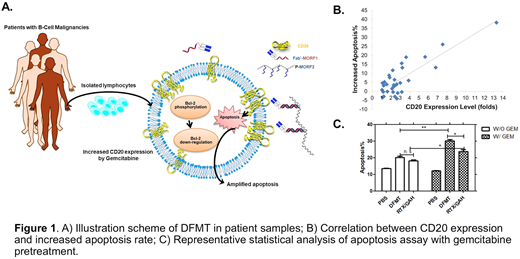
Methods Malignant B cells were isolated from patients diagnosed by the hematologic malignancies service at the Huntsman Cancer Institute. The majority of isolates were from CLL patients. CD20 expression of each sample was evaluated by flow cytometry. DFMT consisting of two nanoconjugates was used to treat the cells in consecutive administration: first, cells were incubated with Fab'-MORF1 (bispecific engager, Fab' fragment of RTX conjugated with a morpholino oligonucleotide); after 1 h, a crosslinking effector (CLE) containing multiple copies of complementary morpholino oligonucleotide (MORF2) was added and the cells were continually incubated for another 6-20 h (depending on assays). The CLE can be prepared by conjugation of MORF2 to either N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer precursor or human serum albumin. Crosslinking of CD20 receptors is initiated by the multiple hybridization of MORF1/MORF2 at the cell surface, and results in apoptosis (Fig. 1A). Annexin V and caspase 3 assays were used to quantify B-cell apoptosis. RTX followed by goat-anti-human (GAH) secondary antibody was used for comparison. GAH was added to imitate the function of Fcγ+ immune effector cells for crosslinking of RTX. When enough cells were available, a detailed evaluation of apoptosis induction was performed, i.e. plasma membrane rupture, genomic DNA fragmentation, mitochondrial membrane permeabilization, Bcl-2 inhibition and confocal imaging to document specific biorecognition at the cell surface. In some samples, pretreatment with GEM or 2P-GEM for 48 hours followed by DFMT or RTX/GAH was conducted, and the effect of GEM in enhancing apoptosis induction was evaluated.
Results The efficacy of DFMT was assessed in 44 samples including 35 CLL along with a few other B cell lymphomas such as diffuse large B cell lymphoma (DLBCL), marginal zone lymphoma (MZL), follicular lymphoma (FL), etc. DFMT induced apoptosis in 65.9% of patient samples.
High-risk mutations such as 17p13 and 11q22 deletions, usually considered as poor prognostic factors in CLL, did not hamper the therapeutic efficacy of DFMT treatment. In cases with poor responses, we noted low CD20 expression levels (Fig 1B). We previously showed that pre-treatment with GEM can enhance surface CD20 expression and restore responsiveness to anti-CD20 mAb. Therefore, we pre-treated the low CD20 patient samples with GEM followed by treatment of DFMT, which induced significantly more apoptosis than RTX/GAH control (Fig 1C). In addition to increased target expression (CD20), this superior activity is likely due to synergy between GEM and DFMT, as we have shown that CD20 crosslinking triggered by DFMT stimulates a cascade of apoptotic events that eventually lead to up-regulation of the pro-apoptotic proteins Bax and inhibition of NF-ĸB, which in turn sensitizes cells to GEM.[Li L, et al. ACS Nano 2018]
Conclusion DFMT effectively increased apoptosis of tumor cells from patients with a variety of B-cell malignancies, irrespective of genomic aberrations. The apoptotic response to DFMT was significantly correlated with CD20 expression, and could be enhanced by GEM-induced upregulation of CD20. DFMT alone or in combination with 2P-GEM warrants further evaluation as a therapeutic for refractory B cell malignancies.
Yang:Baston Biologics Company: Membership on an entity's Board of Directors or advisory committees; University of Utah: Patents & Royalties: PCT/US2014/023784 and PCT/US2017/37736. Deininger:Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Blueprint: Consultancy. Shami:Lone Star Biotherapies: Equity Ownership; Pfizer: Consultancy; JSK Therapeutics: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Baston Biologics Company: Membership on an entity's Board of Directors or advisory committees. Kopeček:University of Utah: Patents & Royalties: PCT/US2014/023784 and PCT/US2017/37736; Baston Biologics Company: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal